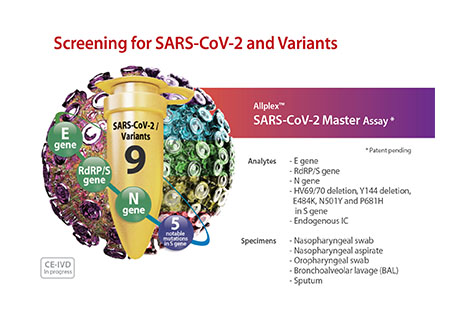
We are pleased to announce the new AllplexTM SARS-CoV-2 Master Assay from Seegene today.
In addition to the wild-type genes E, S, RdRP and N of the coronavirus, this innovative assay also includes the variants HV69/70 deletion, Y144 deletion, E484K, N501Y and P681H of the S gene, all in one tube for easy and simultaneous variant monitoring.
This new assay also includes an endogenous control to minimize false negative results and thus guarantees a reliable result.
The assay has already been validated for various sample types: Nasopharyngeal swab, Nasopharyngeal aspirate, Oropharyngeal swab, Bronchoalveolar lavage (BAL) and Sputum.
The assay has already been validated for various sample types: Nasopharyngeal swab, Nasopharyngeal aspirate, Oropharyngeal swab, Bronchoalveolar lavage (BAL) and Sputum.
We offer you this precise and complete Real-time Multiplex Full Screening Assay with the five important S-gene variations in addition to the four target genes of SARS-CoV-2, at the same conditions as our AllplexTM SARS-CoV-2 Assay. The new AllplexTM SARS-CoV-2 Master Assay is expected to be available from the beginning of April 2021.
For further information, please click on the flyer below or contact us directly.

Social Links